Tick-Tock! Building Molecular Clocks With Tanya Zelevinsky
The experimental physicist uses quantum control of molecules to study fundamental science.

Tanya Zelevinsky knows a thing or two about cold. The experimental physicist who studies atoms and molecules near absolute zero (that’s over –400 °F) spent her early years in a Siberian research town where subzero winters were the norm.
Zelevinsky grew up among scientists and engineers, her parents included, who sparked an early interest in science. That led her to MIT and an introduction to atomic physics during a summer program at Los Alamos National Lab. After receiving her PhD from Harvard, she joined JILA, a research institute in Colorado, as a postdoc.
While there studying atomic clocks, Zelevinsky had an idea: to make ultracold molecules from ultracold clock atoms.
Jun Ye, Zelevinsky’s mentor at JILA, was impressed from the start. “When she joined my group, she had never worked with cold atoms before. But she jumped right in and became a leader in the field in just a few short years,” he said.
In 2008, she joined Columbia, launching a new era of atomic and molecular physics at the university. She and the ZLab, as her group is known, build and study molecular clocks. These clocks rely on pairs of cold atoms whose quantum states can be precisely controlled.
The devices aren’t just for keeping time. Zelevinsky is building clock molecules to explore the universe—all on tabletops in Pupin Hall.
Keeping Time and Asking Questions
Atomic clocks are currently the most precise timekeeping devices ever built—the reference clock at the National Institute of Science and Technology, for example, will gain or lose a second just once in 300 million years. Atomic clocks define our concept of time and enable precision measurements and technologies like GPS. Their “ticking” comes from the movement of electrons as they shift between orbitals that circle the atom—a so-called electronic state.
Clock molecules are diatomic, made of two atoms bound together that vibrate and rotate relative to each other. The motion of these vibrating and rotating quantum states serve as “ticks” that can, at least theoretically, last longer than the electronic states that enable atomic clocks. Whether molecular clocks will ever beat the temporal precision of atomic ones remains to be seen, said Zelevinsky. But she has another application in mind: using clock molecules to ask fundamental questions about physics and the universe.
One mystery is gravity. From entire galaxies down to microscopic scales, gravity works just as Isaac Newton predicted hundreds of years ago. But at smaller scales? No one knows, said Zelevinsky. There are similar conundrums regarding so-called dark matter, a gravity-related component of the universe that researchers haven’t found a place for in existing theories, and about the difference between matter and antimatter—these should exist in equal proportions, but signs of antimatter have been few and far between.
There are two main approaches to experimentally tackle these problems. One is to build massive, high-energy particle accelerators. These can yield powerful insights, said Zelevinsky, but they are expensive and hard to scale up. The other option is to go small with compact setups like clocks, which can be built on tabletops in labs and allow researchers to test ideas at lower cost and with more flexibility.

Both atomic and molecular clocks can be sensitive to unexpected new forces, so researchers can look for anomalies in their ticks that classic Newtonian physics and electromagnetism can’t explain. Because they vibrate, molecular clocks could be more affected than atomic ones, said Zelevinsky. But first, you have to build them.
Building Clocks, and Connections
One way to build molecular clocks is to use lasers to cool individual atoms close to absolute zero, at which point any motion left is quantum mechanical. Once a few million atoms are cold enough, another laser coaxes them to form pairs that are then trapped in lattices made of light. These molecules are now ready for an experiment.
The ZLab has been focused on making clocks out of strontium (Sr), a laser-cooling-friendly element. Their clocks have used its most common isotope, 88Sr; Zelevinsky plans to start using forms that have different masses, which will be affected differently by yet-unknown gravity-like forces.
But, not all atoms can be cooled. As an alternative, the ZLab is one of only a handful around the world working to laser cool molecules directly. This is challenging because three dimensions need to be accounted for—translation, vibration, and rotation—instead of just one, she said. Recently, they cooled calcium monohydride, a molecule made of calcium and hydrogen, in one dimension. They plan to try for all three this summer.
With the help of yet another laser, diatomic molecules can also be broken down into two individual atoms. Such dissociation may help researchers work with atoms that cannot be cooled directly, like hydrogen. If the Zlab can successfully cool calcium monohydride, they want to then split off its hydrogen atoms, in order to study what secrets the universe’s most abundant element might hold.
Zelevinsky is currently constructing clocks for fundamental science in her own lab at Columbia and is working with collaborators from the University of Chicago and the University of Massachusetts on a device housed at Argonne National Laboratory. At the moment, she’s at the Niels Bohr Institute in Copenhagen, sharing her knowledge about cold molecules with European colleagues.
Over the years, she’s mentored more than 60 students and postdocs. “Tanya gave me a ton of freedom to pursue high-risk, high-reward ideas,” said Mickey McDonald. During his PhD in the ZLab, he wanted to know why dissociating molecules formed blurry clouds, which required an imaging system that could take sharper pictures. “She gave me the time and resources to pursue something for the sake of my own curiosity, and without a guarantee that the results would lead to anything interesting,” he recalled.
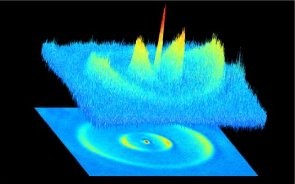
The results were serendipitously spectacular. As the molecules were broken apart, they could see quantum waves in the atoms’ trajectories. McDonald won the American Physical Society’s DAMOP thesis prize for the work, which Zelevinsky counts as one of her proudest moments.
When she’s not in the lab, Zelevinsky keeps busy with her two children, 13 and almost 10, who have been a part of the ZLab since the beginning. She has no idea if they will follow in her footsteps, “but I’m watching with interest,” she said.
It’s now been almost 15 years at Columbia, and other researchers with an interest in ultracold science have since joined Zelevinsky in New York. There’s still a lot to do with molecular clocks, but she is seeing to it that this cold science keeps heating up.
